WHO Recommends Saudi Arabia’s Public Health Laboratory for Recognition as National Influenza Centre
WHO encourages the public health laboratory to continue to contribute to regional and global influenza surveillance, enhancing its involvement in GISRS.
A WHO mission to Saudi Arabia has found that the country’s public health laboratory operating within the Public Health Authority has fulfilled the requirements for WHO to recommend recognition of the laboratory as a national influenza centre for Saudi Arabia.
The public health laboratory plays a critical role in surveillance and support of public health informatics to rapidly detect and monitor infectious diseases, outbreak investigations and scientific analysis of known, novel and emerging diseases.
The laboratory designated by the Ministry of Health as the national influenza laboratory in 2017 has been expanding its services for the subtyping of influenza and other respiratory viruses to participate in the new integrated surveillance programme in the country. This in addition to the COVID-19 laboratory that acted as a reference laboratory through the pandemic for COVID-19 testing. There is also a genome laboratory with a very wide range of platforms and advanced technologies for genome sequencing.
To address public health priorities, public health laboratories are expanding their services to include active surveillance programmes for antimicrobial resistance, food safety, environmental health and high containment facilities. They are also offering a range of programmes and functions, including a WHO-accredited national polio laboratory to support global polio eradication activities, a vaccine-preventable diseases laboratory with reference diagnostics and surveillance for measles, mumps, and rubella, and a laboratory for measles genotyping.
The aim of the mission was to assess the compliance of the laboratory through the WHO terms of reference to become a WHO-recognized national influenza centre, as well as a formal member of the WHO Global Influenza Surveillance and Response System (GISRS).

Excellent quality and cooperation
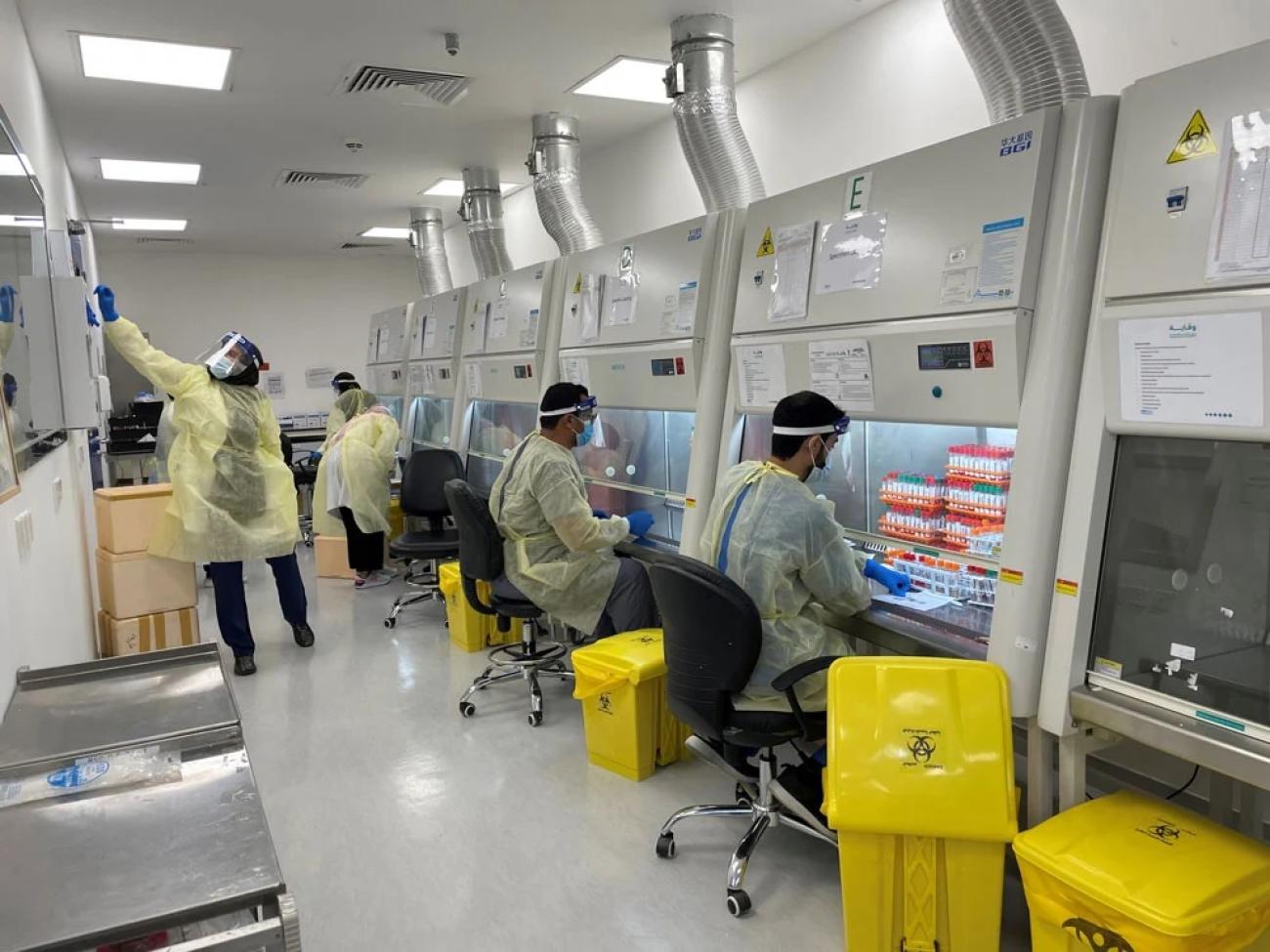
The laboratory has an excellent quality and biosafety management system. Photo: WHO/A. BarakatSaudi Arabia’s public health laboratory was found to have facilities that can accommodate the activities of virological surveillance of circulating influenza viruses and other respiratory pathogens. The facilities are well-maintained, and the equipment is modern and functional. The laboratory has an excellent quality and biosafety management system with procedures in place for processing specimens from start to finish. The laboratory has adequate capacity for the storage of clinical specimens and propagated viruses. The influenza unit is also adequately staffed to perform registration, processing, testing, and storage of specimens collected for influenza surveillance and reporting.
The virology laboratory has Biosafety Level 2 plus (BSL-2+) facility, real-time PCR capability, facilities for virus isolation and for serological studies, and sequencing facilities.
The laboratory has already contributed to the regional influenza network and to GISRS by sharing clinical specimens with the WHO Collaborating Centre for Reference and Research on Influenza in London. The laboratory has regularly participated in the WHO External Quality Assurance Project on the detection of influenza viruses by PCR since 2018 and demonstrated excellent performance in 2021. The laboratory continues to provide regular reports on influenza surveillance data to WHO.
WHO encourages the public health laboratory to continue to contribute to regional and global influenza surveillance, enhancing its involvement in GISRS. WHO and its collaborating centres for influenza are committed to continuing to provide technical advice, assistance, influenza reagents and training to the national influenza centre.
Related links
Learning from Saudi Arabia’s COVID-19 response amid its health sector transformation


